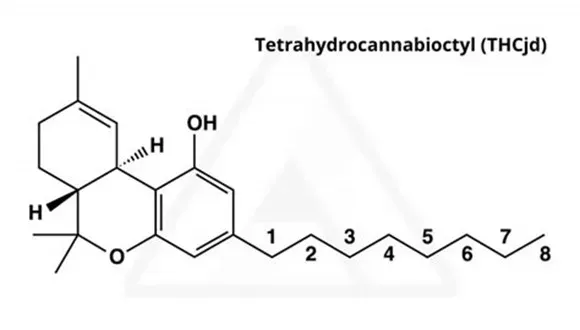
THC-JD
THC-JD, which is also referred to as Δ9-THC-JD and octyl THC, is a largely unexplored cannabinoid naturally present in the hemp and cannabis plants. By contrast to conventional Δ9-THC, THC-JD, has a side hydrocarbon chain consisting of eight carbon atoms. This hydrocarbon chain is saturated, so no double or triple bonds are present between any of the carbon atoms. In addition, there are no heteroatoms such as oxygen, sulfur or nitrogen found on the eight carbon side chain. Conventional Δ9-THC has a five carbon saturated chain attached which is also free from any heteroatoms. The structures of Δ9-THC and Δ9-THC-JD, are shown for contrast below.
Δ9-Tetrahydrocannabinol
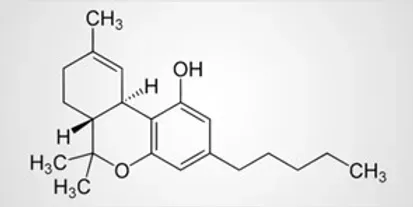
THC-JD is present at very low concentrations in the cannabis and hemp plants. Obtaining the material in quantity and in pure form is currently quite challenging. A small number of companies are attempting to make this elusive molecule available. Cannabis aficionados may well ask if it is a task worth pursuing.
THC-JD, which is also referred to as Δ9-THC-JD and octyl THC, is a largely unexplored cannabinoid naturally present in the hemp and cannabis plants. By contrast to conventional Δ9-THC, THC-JD has a side hydrocarbon chain consisting of eight carbon atoms. This hydrocarbon chain is saturated, so no double or triple bonds are present between any of the carbon atoms. In addition, there are no heteroatoms such as oxygen, sulfur or nitrogen found on the eight carbon side chain. Conventional Δ9-THC has a five carbon saturated chain attached which is also free from any heteroatoms. The structures of Δ9-THC and Δ9-THC-JD are shown for contrast below.
Δ9-Tetrahydrocannabinol

Tetrahydrocannabioctyl (THC-JD)

THC-JD is present at very low concentrations in the cannabis and hemp plants. Obtaining the material in quantity and in pure form is currently quite challenging. A small number of companies are attempting to make this elusive molecule available. Cannabis aficionados may well ask if it is a task worth pursuing.
One aspect of THC-JD which is very engaging is the potential for adding a new psychoactive substance to the existing group which may arguably be legal. Octyl THC obtained from hemp plants containing less than 0.3% by weight of Δ9-THC after drying is likely to be legal under federal law. This natural compound may be covered by aspects of the Agriculture Improvement Act of 2018, also known as the “Farm Bill”. This would allow THC-JD to be sold throughout much of the nation.
Let us now ask the really important question. Does THC-JD cause people to experience a marijuana like high?
To determine if octyl THC is likely to allow users to experience a marijuana like high, we must determine if this material can bind to the CB1 receptor found on cell membranes of neurons located in the central nervous system. Further, we must explore the degree to which THC-JD binds to the CB1 receptor protein.
The binding of a material to a receptor is quantified by measuring the EC50 value. Another name for this value is Ki. The EC50 value is the concentration of a material in solution that is sufficient to bind to half of the available receptors. Depending on the specific receptor under study, these concentrations are measured in micromoles per milliliter or nanomoles per milliliter.
A lower Ki value corresponds to a lower concentration in solution of the substance being tested that is required to bind to half of the receptors. Stated another way, lower Ki values indicate a greater tendency of the test substance to bind to the receptor protein. Lower Ki concentrations indicate that the test substance has a higher affinity for the receptor protein. Higher affinity is often connected to enhanced potency and effectiveness.
It would be very useful to be able to look up an EC50 value for octyl THC and compare it to the known value for Δ9-THC. Unfortunately, the experiments that would allow scientists to calculate an EC50 value for octyl THC have not yet been performed. No value for the Ki concentration of THC-JD in solution can be found in the technical literature at the time of this writing. To gain an understanding of the binding affinity of octyl THC to the CB1 receptor, we will have to utilize model compounds as a proxy for octyl THC. Fortunately, several excellent model compounds have been studied.
Below is a table which shows the measured Ki concentrations for conventional Δ9-THC along with some simple analogs of the parent molecule. Δ9-THC has a five carbon saturated side chain. This is also referred to as a pentyl chain. The measured Ki for Δ9-THC is 40 nanomoles per milliliter. At this concentration of THC in solution, half of the CB1 receptors are bound to a molecule of Δ9-THC.
At the top of the table is an entry for an analog of Δ9-THC which has a three carbon chain attached rather than a five carbon chain. This is also called a propyl chain. The Ki value obtained for the propyl derivative of THC at the CB1 receptor is 75.4 nanomoles per milliliter. It requires about twice as much of the propyl THC analog in solution to reach a condition where half of the CB1 protein receptors are occupied.
Further down in the table is an entry for a Δ9-THC analog with a four carbon chain attached. This is also called a butyl chain. The measured EC50 for the butyl analog of THC is 15 nanomoles per milliliter. This is considerably less when compared to the EC50 measured for Δ9-THC. The butyl analog should be 2.67 times more powerful when compared to Δ9-THC. This measure of potency was calculated by dividing the EC50 for Δ9-THC by the EC50 for the butyl analog.
At the bottom of the table is an entry for a Δ9-THC analog with a seven carbon chain attached. This is also called a heptyl chain. The measured Ki for the heptyl analog at the CB1 receptor is 1.2 nanomoles per milliliter. This suggests that the heptyl analog is 33.3 times as potent as ordinary Δ9-THC. As the hydrocarbon side chain is extended, the trend is strongly directed toward increased potency. Extending the side chain by one additional carbon may cause the potency to increase still further when compared to the heptyl analog.
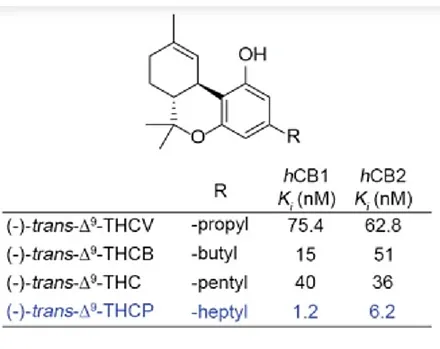
Based upon an examination of model compounds which mimic Δ9-THC, the octyl analog should be a very potent activator of the CB1 receptor in the central nervous system. In answer to the question posed earlier, THC-JD will definitely get you high. It remains for scientists and hobbyists who enjoy cannabis to fill in all of the specific details.
Article written by:
Dr. Leonard Haberman
Chief Science Officer-
Herbalxchange, LLC.